Содержание
Рынок всегда смотрит в будущее, и это ключевой момент для понимания тенденций изменения рыночных индексов. А может быть, можно получить прибыль и из хаоса, даже если цены акций ведут себя абсолютно случайным образом? Эта теория получила широкое распространение среди теоретиков биржи в 50-х годах, когда расчеты на первых компьютерах показали ее соответствие поведению рынка акций. Последующее, более глубокое изучение биржевых процессов выявило ее недостаточность, но об этом мы расскажем позже.
- В течение нескольких дней при большом объеме торговли цена акций падает, «оторвавшись» от уровня поддержки.
- Можно сказать что нисколько не пожалел, объясню почему…
- Нужно постоянно следить за такими акциями и чувствовать все их движения, тщательно изучая новости компании и коррелируя изменение цены акций с общим поведением рынка.
- Эти виды спорта приносят огромное удовольствие, но считаются очень опасными, потому что многие спортсмены недооценивают степень риска и действуют бесшабашно.
- Угадать момент прорыва флажка трудно, и трейдерам рекомендуется ставить «стоп» на покупку в случае падающего флажка чуть выше его верхней границы.
Безусловно, рынок будет колебаться, но почему-то годовая тенденция. Изменения рыночных индексов во многом коррелирует с январским барометром. Для уменьшения числа подгоняемых параметров предложен другой метод определения точек покупки и продажи акций. Для этого вычисляют динамическое среднее от этого осциллятора, (например по 5 — 9 дням) и полученную линию — она называется сигнальной линией SIG — наносят на тот же график.
Кроме того, мониторинг ситуации позволит вам понимать, какие сигналы являются ложными, а какие приведут к устойчивому тренду. Не спешите, пользуйтесь выбранной вами стратегией, ведите статистику, анализируйте каждое изменение на рынке. Первая прибыль станет самым приятным бонусом и придаст вам уверенности. Так как Бартон Биггс сам является владельцем хедж-фонда, основной акцент сделан именно на тенденции развития этого вида торговли.
Шаг 6. Получение 1-ой прибыли от биржевой игры
Он как известно технический трейдер, а не фундаментальный. Особый интерес книга вызовет у тех, кто, как и Джесси Ливермор, начинает торговать на бирже, имея минимум средств и желание разобраться в «кухне» биржевой торговли, чтобы выиграть свой миллион. Торговля на бирже – валютной, фондовой или товарной позволяет не только уберечь деньги от инфляции, но и принести немалый доход. Но лишь при овладении некоторыми навыками и знании правил и секретов.
Покупка акций рассматривается не просто как набор цифр и тикетов, а возможность стать совладельцем компании. В книге говорится о неизбежных падениях и восхитительности сложных процентов, которые дают возможность расти. Рассмотрены принципиальные отличия пассивного инвестирования в отличии от активного (спекуляций). Разобран метод недооцененных акций и метод глубокой стоимости. В приложении показаны результаты товариществ Баффетта по сравнению с ведущими трастами и взаимными фондами с 1956 по 1970 г.
Рассматривая медицинские компании, нужно отметить, что их доходы (и поведение акций) во многом определяются государственной политикой медицинского страхования. Для небольших биотехнологических и фармацевтических компаний ключевым вопросом является не разработка новых лекарств, а получение разрешения комиссии по пищевым продуктам и лекарственным препаратам FDA . Прежде чем FDA даст добро на массовое производство и продажу этих лекарств, они должны пройти многолетние лабораторные и клинические испытания, которые стоят много миллионов.
ТОП 25 лучших книг для начинающих трейдеров
Важно понимать, что новичку нужно для начала изучить хотя бы основные правила биржевой торговли, иначе он просто не справится самостоятельно. Минусы у биржевой торговли онлайн есть — нельзя видеть реальные котировки и можно столкнуться с брокерами-мошенниками. Впрочем, если вы помните фильмы о бизнесе, мошеннические схемы могут реализовывать и брокеры, работающие на реальных торговых площадках. Если раньше трейдерам, биржевым игрокам, приходилось собираться в одном здании или вести торговлю в телефонном режиме, то теперь все чаще игра ведется через интернет. Это очень удобно, все можно делать дома, следя за последними новостями и биржевыми сводками.
Скажем, потеря 50% этой доли не должна изменить стиль вашей жизни. Доля инвестированного капитала, вложенного в надежные компании, тоже должна быть в этом случае больше. Деньги, предназначенные для трейдинга, при такой ситуации надо рассматривать как деньги для развлечения — полная их потеря в случае серии неудач не должна заметно огорчить вас. Как видно из этого перечисления, требования к компаниям, представленным на нью-йоркской бирже, гораздо более высокие, чем к компаниям на рынке NASDAQ. Отметим, что некоторые фирмы-гиганты предпочитают оставаться на рынке NASDAQ. Это связано отчасти с тем, что регистрация на нью-йоркской бирже требует значительной отчетности и дополнительных затрат.
Брокерские комиссионные и другие накладные расходы еще больше усугубляют ситуацию. Практически, игра на понижение не так сложна, как может показаться из описания. Вам нужно выбрать «плохие» акции, определить предел, с которого вы готовы начать игру, и заказать брокеру (можно через компьютер), sell short, скажем, 100 акций XXX с пределом 10. Если акции доступны и их цена изменится согласно правилу uptick, то ваша позиция для игры на понижение будет открыта. Брокер нашел компанию ABC, акции которой в самом деле выросли в 4 раза. Далее он звонит вам и нагло врет, что звонил 4 месяца назад с рекомендацией купить эти акции.
Выбор брокера
Вы, вероятно, уже немного устали, но, к сожалению, успех на бирже не может быть достигнут без большого труда, поиск хороших акций напоминает добычу радия из урановой руды. Приходится просматривать не десяток цифр, которые представлены в этой книге, а тысячи. И все для того, чтобы найти одну-две компании, заслуживающие внимания, но ваш труд будет всегда вознагражден. Объемы продаж и прибыли компании быстро растут, долги уменьшаются, наличный капитал увеличивается. Начинается выплата заметных дивидендов инвесторам.
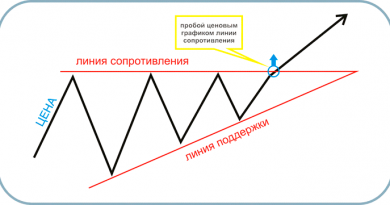
Число дней, по которым проводится суммирование может быть произвольным, ибо при использовании этого индикатора важно только его изменение, но не абсолютная величина. Когда же все-таки продавать акции, если на графике появится такая фигура? Обычно рекомендуют поставить «стоп» на продажу чуть ниже линии «шеи» после правого плеча. Почему бы не продать сразу после вершины правого плеча, как логично было бы предположить? Дело в том, что данное плечо должно еще сформироваться, ведь небольшое падение с вершины плеча может быть обусловлено только дневными колебаниями курса акций.
Литература, которую советуют профессионалы
Это оказывает неоценимую поддержку участникам рынка с любым опытом – от новичка до профессионала. Представленная публикация поможет новичкам разобраться в основах торговли на бирже. В итоге начать биржевую игру можно будет с большей уверенностью.
Как выбрать компанию для инвестирования
Книга от автора знаменитой книги, названной “библией” технического анализа. Спустя 5 лет Джон выпустил данную книгу, которая также стала пользоваться успехом. Книга дополняет прошлую многочисленными примерами.
Эта книга о том, как зарабатывать деньги на изменениях курсов валют. Прочитав ее, вы узнаете, как устроена мировая финансовая система и как обычному человеку стать частью… Иногда возникает желание отвлечься, хочется почувствовать, что ты словно живёшь какой-то другой жизнью. Можно забыть о настоящем и погрузиться в то, что происходит на страницах книги.
Деятельность нашей компании лицензирована в Европейском cоюзе и регулируется единым финансовым законодательством ЕС, в том числе новейшей директивой «O рынках финансовых инструментов» . Лицензия (№ 4.1–1/46) является действительной во всех 27 странах – участницах Евросоюза. Решение о продаже акций на определенной цене вы принимаете в спокойной обстановке, при домашнем анализе, а не во время работы биржи, когда цены меняются непрерывно, вселяя то надежду, то ужас. «Стоп» реализует взвешенное решение, не опоздав ни на секунду. При изменении цены акций обязательно передвигайте «стоп» только в сторону уменьшения возможных потерь и сохранения большей прибыли.
Для новичков такие условия могут стать ловушкой, средства «зависнут» на депозите на непонятное время. Как вы увидели по нашей инструкции для новичков — выбор брокера для биржевой торговли невероятно важен! Вам нужно будет указать на сайте выбранного брокера адрес электронной почты, ФИО, телефон для контактов.
Как происходит про биржевые инвестиционные фонды etf для начинающих акциями и кто определяет их цену? Первичный инвестиционный банк покупает акции у компании по одной цене, а распределяет по более высокой, которая является первой рыночной ценой акций. Это называется первичным распределением акций (первичный рынок), которое недоступно рядовым инвесторам. После первичного распределения акции попадают на вторичный рынок, где брокерские фирмы осуществляют продажу акций всем желающим. Изучение этого рынка и является целью данной книги. Цена акций на вторичном рынке уже определяется спросом и предложением и устанавливается дилерами рынка.
На акциях, цены которых случайно блуждают, в среднем сделать прибыль нельзя, как это было показано в предыдущем разделе, а с акциями Джима и Майкла прибыль сделать можно. Выбор этих «аналитиков» любят сравнивать с выбором профессионалов, подчеркивая, что иногда профессионалы оказывались позади. Но вернемся к главной цели нашей книги — к рассказу об игроке на бирже, принимающем самостоятельные решения. Такому игроку нужен так называемый дискаунт-брокер, который не дает никаких советов и берет за свои услуги минимальные комиссионные. Величина комиссионных определяется качеством услуг и количеством клиентов у фирмы. Есть брокерские фирмы, которые производят покупку и продажу акции только с помощью компьютера через Интернет, — их комиссионные минимальны.
https://fx-strategy.info/ и другие книги Лотмана будут не менее интересными. Нам всегда хочется верить в рост, в том числе и на рынках. И если в жизни такая вера часто помогает, то на бирже это грозит провалами. Ведь все должно начинаться с адекватной оценки рыночной ситуации и собственных возможностей. Для того, чтобы верить в рост тренда, надо чтобы эта вера была основана на чем-то ощутимом и основательном. Везение брать в расчет не будем, так как мы торгуем по техническому анализу, а в теханализе, как известно, “по-барабану” куда пойдет рынок – главное защита и заявки стоп-лоссы по убыткам и take-профитам.
В этой точке начнут продажу акций многочисленные неопытные игроки, которые купили акции по 25 долларов и прождали все это время, чтобы выйти из игры хотя бы без потерь. Рост акций остановится, и существует очень большая вероятность, что акции снова будут торговаться в диапазоне 24 — 25 долларов. Вероятность тем больше, чем более длительное время акции продержались в этом диапазоне вначале, ибо с этим связано число игроков, купивших акции по данной цене.
